Guangbo Liu1, Huasen Lu1, Yingshuang Xu1, Qinghao Quan1, Honghao Lv1, Xuejing Cui1, Jie Chen1, Luhua Jiang 1,*, R. Jürgen Behm2,*
1 College of Materials Science and Engineering, Qingdao University of Science and Technology, Qingdao266042, P. R. China
2 Institute of Theoretical Chemistry, Ulm University, Ulm 89069, Germany
ABSTRACT
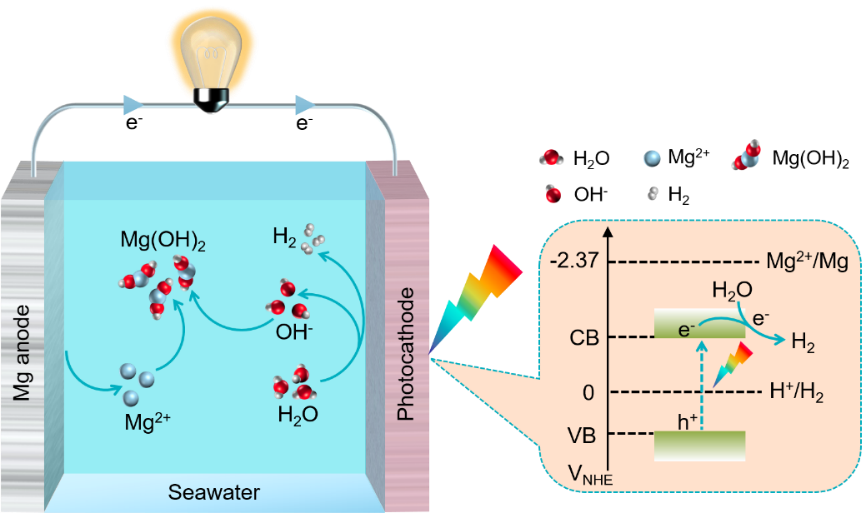
A seawater-electrolyte Mg/H2O battery, converting chemical energy of Mg into electricity and hydrogen via anodic/chemical oxidation of Mg and cathodic reduction of water, combines electricity generation and H2 production, thus is not only ideal power source for marine equipment, but also intriguing on-line/offshore H2 generator for fuel cells. Nonetheless, it substantially suffers from the slow kinetics of hydrogen evolution reaction at the cathode. Herein, we propose a proof-of-concept design of a photo-assisted Mg/H2O battery with simulated seawater (0.5 M NaCl, pH = 6.8) as the electrolyte, and a CuSCN/Cu2O photocathode is applied to promote the hydrogen evolution reaction kinetics and battery performance. Under illumination, the CuSCN/Cu2O reveals a photocurrent density of up to 4.32 mA cm-2 at 0 V vs. reversible hydrogen electrode, over 1.7 times higher than that of Cu2O. A photo-assisted Mg/H2O battery is demonstrated with a peak power density of 1.18 mW cm-2 and a H2 production rate of 0.56 mL cm-2 min-1 under illumination, which is 3.6 and 9.6 times higher than those obtained in the dark. This novel strategy of a photo-assisted Mg/H2O battery enables the simultaneous conversion of photo- and chemical energy into electric energy and hydrogen from seawater.
KEYWORDS: Photo-assisted Mg/H2O battery; electric energy; hydrogen evolution; CuSCN/Cu2O; photocathode.
*Email: luhuajiang@qust.edu.cn, juergen.behm@uni-ulm.de
https://doi.org/10.1016/j.cej.2022.140875