Boyang Zhang, Jing Liu*, Wanqing Yu, Xuejing Cui, Luhua Jiang*
College of Materials Science and Engineering, Qingdao University of Science and Technology,
Qingdao, Shandong 266042, China.
Abstract:
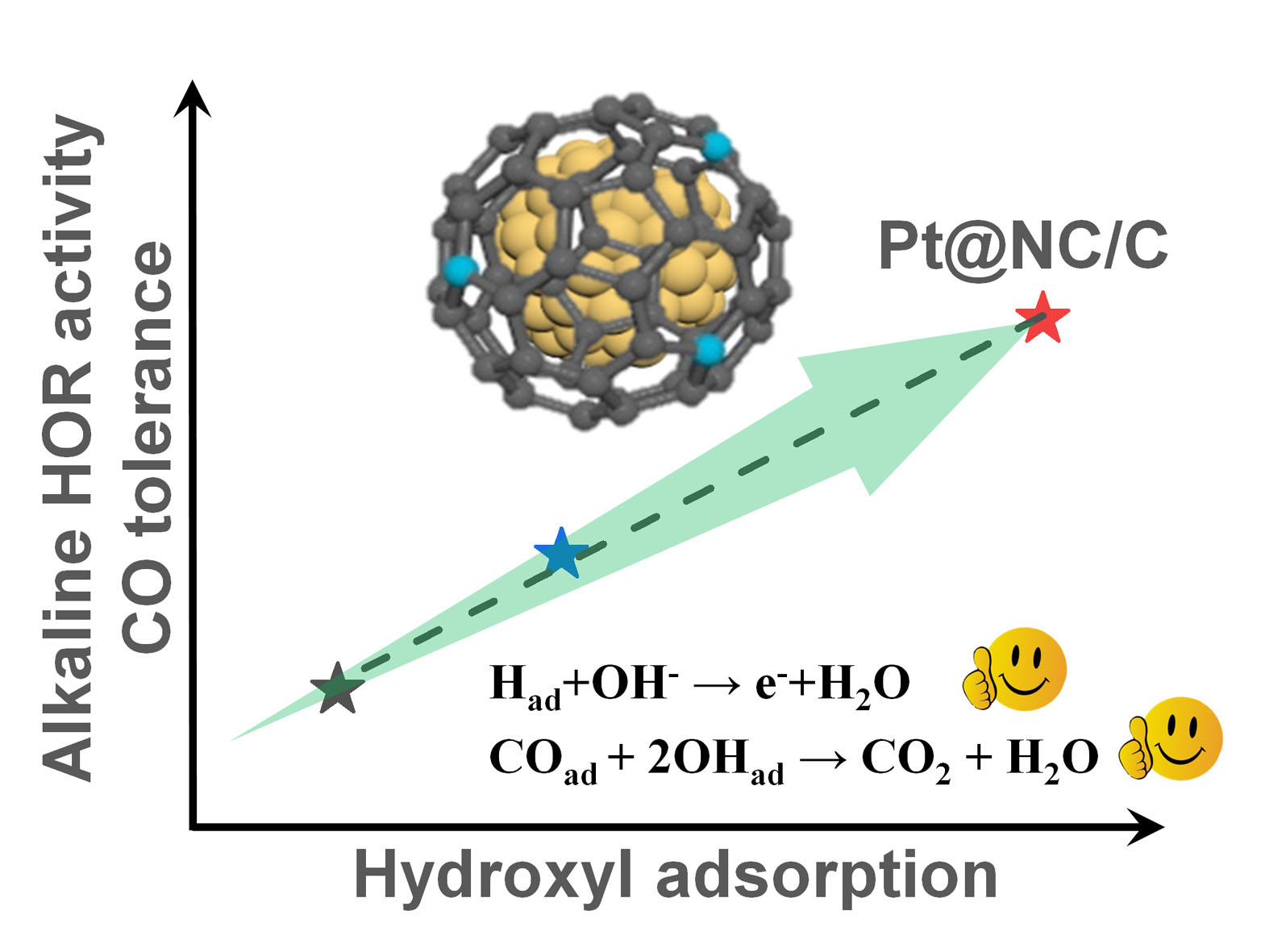
Improving the CO-tolerant ability of Pt-based electrocatalysts is crucial for fuel cells fed with industrial byproduct hydrogen. Herein, we present a novel core-shell structured Pt@NC/C catalyst consisting of ultrafine Pt nanoparticles (1.63 nm) as the core and ultrathin nitrogen doped carbon layers (0.36 nm) as the shell. The catalyst exhibits excellent alkaline hydrogen oxidation reaction (HOR) activity with a mass activity of 187 A g-1Pt and a specific activity of 0.20 mA cm-2Pt, which are 1.3 and 2.2-folds to the counterpart Pt/C, respectively. More remarkably, it shows good anti CO-poisoning ability. In presence of H2 with 100 ppm CO, the HOR current decreased by 18.22% on 10% Pt/C versus only 4.81% on 10% Pt@NC/C-400. The combined COad stripping, CO oxidation and Zeta potential measurements imply that hydroxyl adsorption is enhanced on the NC covered Pt in 10% Pt@NC/C-400, resulting in accelerated HOR kinetics and improved anti-CO ability. This work provides a facile and feasible strategy to design efficient and CO-resistantto Pt-based catalysts.
Keywords: anion exchange membrane fuel cells; hydrogen oxidation reaction; CO poisoning; platinum catalyst
*Corresponding authors: liuj955@qust.edu.cn (J. Liu); luhuajiang@qust.edu.cn (L. Jiang).