Huimin Chen1, Min Wang1,*, Kaicai Fan1, Linghui Kong1, Honghao Lv1, Wenzhen Li2, Guangbo Liu1, Luhua Jiang1,*
1 Nanomaterials and Electrocatalysis Laboratory, College of Materials and Engineering, Qingdao University of Science and Technology, 53 Zhengzhou Road, Qingdao 266042, P. R. China
2 Department of Chemical & Biological Engineering, Iowa State University, Ames, IA 50011-1098, Unites States.
* E-mail addresses: wmin@qust.edu.cn (M. Wang); luhuajiang@qust.edu.cn (L. Jiang)
Abstract
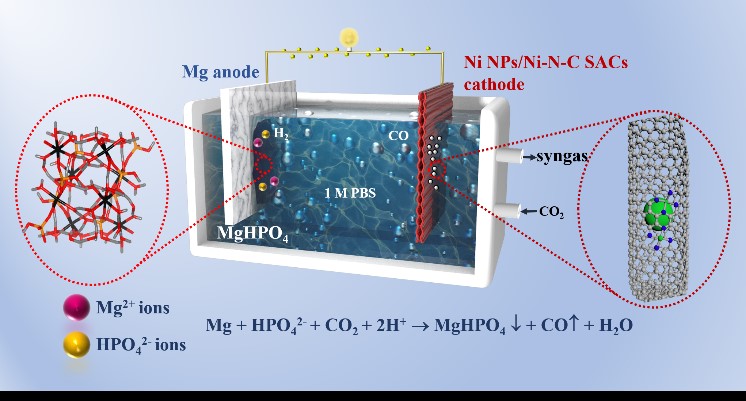
Mg–CO2 battery as a promising strategy for CO2 conversion and utilization is challenging, owing to the sluggish kinetics of CO2 reduction reaction (CO2RR) and the waste of sacrificial Mg anode. Herein, by elaborately designing a highly selective Ni NPs/Ni-N-C SACs electrocatalyst towards CO2RR and rationally optimizing the electrolyte, an effective aqueous-phase Mg–CO2 battery is demonstrated to generate not only electricity, but also syngas with CO from the CO2RR and H2 from the chemical oxidation of Mg, together higher-valued MgHPO4 phosphate. Notably, the output of electricity, the proportion of CO/H2 in syngas, and the yields/crystalline quality of MgHPO4 can be well regulated by manipulating the operation parameters. The Mg–CO2 battery generates a favorable open-circuit voltage of 1.44 V with a peak power density of 2.91 mW cm-2. Under the optimal condition, the Ni NPs/Ni-N-C SACs catalyst displays both a high FECO of 98.12% at –0.573 VRHE and a high CO2-to-CO current of –20.73 mA cm-2 at –0.973 VRHE, owing to the synergistic effect between Ni particles and Ni-N moieties. The proposed Mg–CO2 battery provides an intriguing approach for CO2 conversion and utilization in off-grid, with the advantage of co-production of higher-valued chemicals at both electrodes.
https://doi.org/10.1021/acssuschemeng.2c07631