Yan Zhao, Shixu Song, Jing Liu, Xuejing Cui, Luhua Jiang*
Nanomaterials & Electrocatalysis Laboratory, College of Materials Science and Engineering, Qingdao University of Science and Technology, Qingdao 266042, P. R. China
*Email: luhuajiang@qust.edu.cn
Abstract
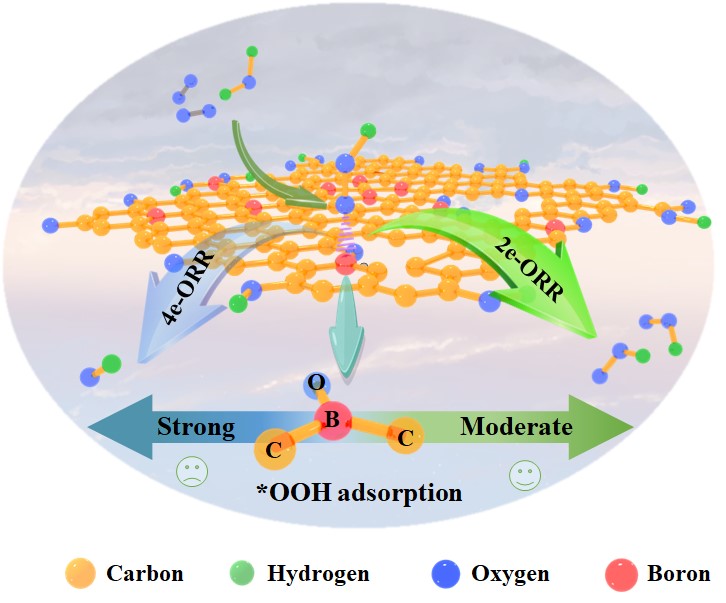
Distributed production of hydrogen peroxide (H2O2) via 2e-oxygen reduction reaction (ORR) has gained significant attention as a green and safe approach. In this work, black liquor-derived carbon is for the first time functionalized as an electrocatalyst for efficient H2O2 production. The co-functionalized porous activated carbon (C3-O3-B10) optimized with oxygen and boron exhibits high activity with almost zero overpotential, satisfactory H2O2 selectivity (77%) and good stability. Mechanism investigations reveal that C3-O3-B10 favors catalyzing a 2e-ORR pathway both thermodynamically and kinetically, with a low Tafel slope of 65 mV dec-1, fast charge transfer ability, and low apparent activation energy of 6.7 kJ mol-1@0.4VRHE, owing to the presence of abundant active C=O and B-C2-O functional groups. This work demonstrates that the functionalized black liquor-derived carbon is a reliable and sustainable electrocatalyst in the production of H2O2, which provides new clues for the utilization of black liquor waste resources in the field of energy conversion and storage.
Keywords: Black liquor-derived carbon; functionalized carbon; oxygen electroreduction reaction; hydrogen peroxide production; oxygen and boron co-doping.
https://doi.org/10.1016/j.jallcom.2023.171264