Min Wanga#, Huimin Chena#, Min Wangb, Jinxiu Wanga, Yongxiao Tuo*c, Wenzhen Lid, Shanshan Zhoua, Linghui Konga, Guangbo Liua, Luhua Jiang*a, Guoxiong Wang*e
a Nanomaterials and Electrocatalysis Laboratory, College of Materials and Engineering, Qingdao University of Science and Technology, 53 Zhengzhou Road, Qingdao 266042, P. R. China
b State Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, 1295 Dingxi Road, Shanghai 200050, P. R. China
c State Key Laboratory of Heavy Oil Processing, College of New Energy, China University of Petroleum (East China), Qingdao, Shandong 266580, P. R. China
d Department of Chemical & Biological Engineering, Iowa State University, Ames, IA 50011-1098, Unites States.
e State Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023 P. R. China
* Corresponding author. E-mail addresses: yxtuo@upc.edu.cn (Y. Tuo); luhuajiang@qust.edu.cn (L. Jiang); wanggx@dicp.ac.cn (G. Wang)
# These authors contribute equally to this work.
KEYWORDS: CO2 electrochemical reduction; in-situ characterization; heterostructure; selectivity to ethanol; DFT calculations
Abstract:
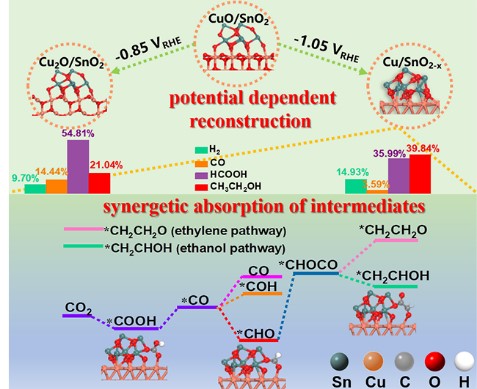
Heterostructured oxides with versatile active sites, as a class of efficient catalysts for CO2 electrochemical reduction (CO2ER), are prone to undergo structure reconstruction under working conditions, thus bringing challenges to understanding the reaction mechanism and rationally designing catalysts. Herein, we for the first time elucidate the structural reconstruction of CuO/SnO2 under electrochemical potentials and reveal the intrinsic relationship between CO2ER product selectivity and the in-situ evolved heterostructures. At -0.85 VRHE, the CuO/SnO2 evolves to Cu2O/SnO2 with high selectivity to formate (Faradaic efficiency of 54.81%). Mostly interestingly, it is reconstructed to Cu/SnO2-x at -1.05 VRHE with significantly improved Faradaic efficiency to ethanol of 39.8%. In-situ Raman spectra and density functional theory (DFT) calculations reveal that the synergetic absorption of *COOH and *CHOCO intermediates at the interface of Cu/SnO2-x favors the formation of *CO and decreases the energy barrier of C-C coupling, leading to high selectivity to ethanol.
全文链接:https://doi.org/10.1002/anie.202306456