Liu Jing a,ƚ, Gao Jie a,ƚ, Wanqing Yu a, Huanwei Ren a, Xuejing Cui a, Xin Chen b,*, Luhua Jiang a,*
a Electrocatalysis & Nanomaterial Laboratory, College of Materials Science & Engineering, Qingdao University of Science & Technology, Qingdao, 266042, China.
b Center for Computational Chemistry and Molecular Simulation, College of Chemistry and Chemical Engineering, Southwest Petroleum University, Chengdu, 610500, China
ABSTRACT:
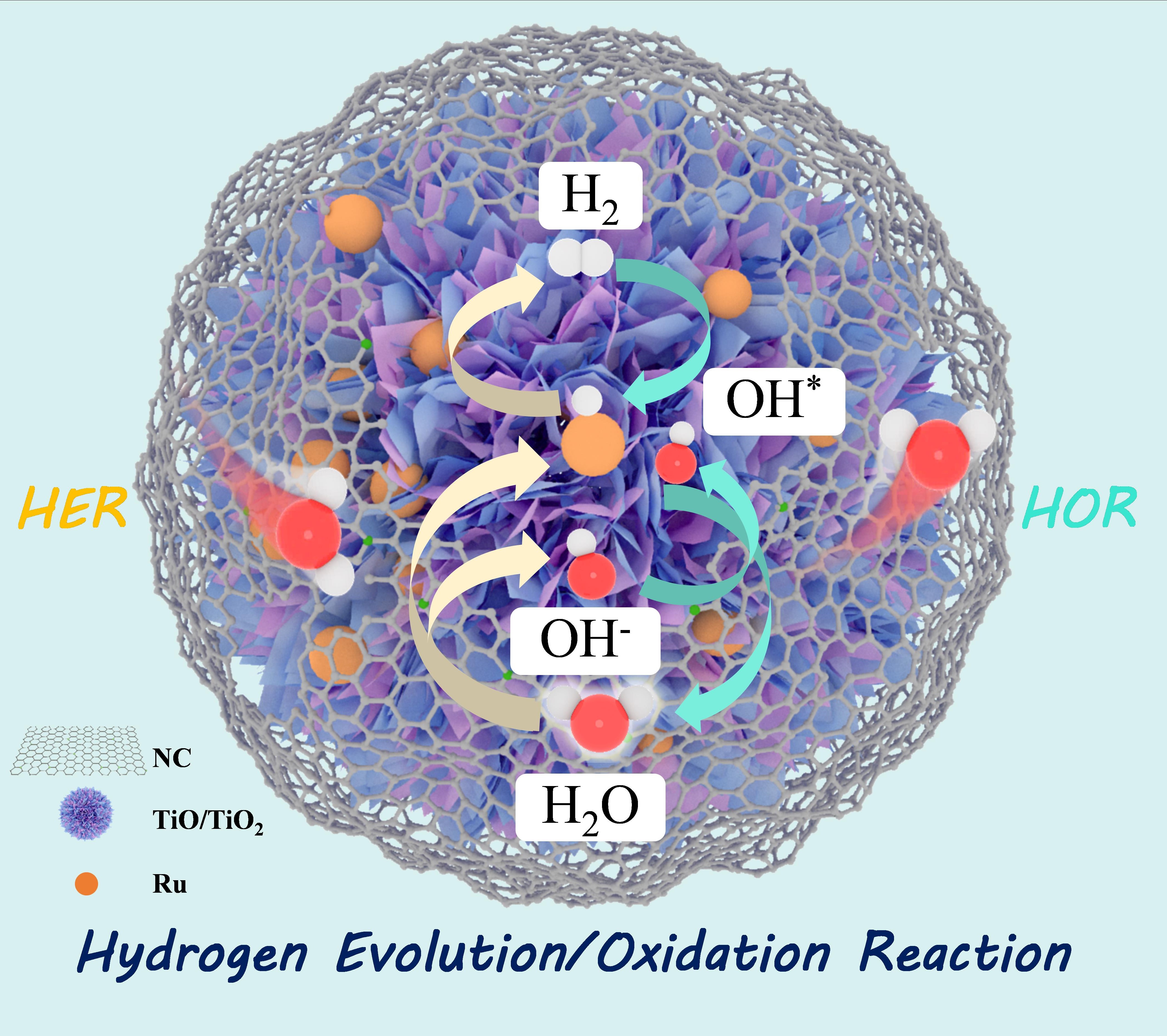
Developing active and stable non-Pt electrocatalysts for hydrogen oxidation (HOR) and evolution reactions (HER) are critical for anion exchange membrane fuel cells and water electrolyzers. Herein, we report a highly active and robust electrocatalyst Ru-TiO/TiO2@NC, in which Ru nanoclusters are sandwiched between TiO/TiO2 nanosheets and nitrogen-doped carbon layers. Taking advantage of both the optimized Ru-TiO/TiO2 interaction and the conductive carbon coating layers, the Ru-TiO/TiO2@NC exhibits both exceptional HOR/HER activity and superior stability, outperforming Pt/C in the alkaline solution. The HOR mass activity reaches up to 107 A gRu-1 at an overpotential of 50 mV, and the specific exchange current density is 0.271 mA cm-2. The HER overpotential at 10 mA cm-2 is only 39 mV, 34 mV lower than required by Pt/C. More importantly, the Ru-based catalyst exhibits excellent anti-oxidation ability by virtue of the unique sandwich structure. Density functional theory calculations discover that the d-band center of Ru in Ru-TiO/TiO2 is downshifted by 0.29 eV compared to Ru-TiO2, decoupling and optimizing the Had/OHad adsorption on Ru, i.e., Had is promoted, while OHad is inhibited and transferred to TiO/TiO2. As a result, (i) the energy required by the potential determining step of HOR/HER is lowered, and (ii) the anti-oxidation ability of the Ru-TiO/TiO2 is enhanced. This work not only addresses the issue of Ru passivation at high anode potentials but also provides an innovative and versatile approach to designing advanced electrocatalysts.
Keywords: TiO/TiO2 heterostructure; hydrogen oxidation reaction; hydrogen evolution reaction; bifunctional catalyst; interface engineering
*Corresponding authors: chenxin830107@pku.edu.cn; luhuajiang@qust.edu.cn
ƚ These authors contributed equally to this work.
https://doi.org/10.1016/j.cej.2023.145009