Guangbo Liua, Shukun Liua, Xiaolei Lia, Honghao Lva, He Qub, Qinghao Quana, Huasen Lua, Xuejing Cuia, Xin Zhoub,*, Luhua Jianga,*, Jieshan Qiuc,*
a College of Materials Science and Engineering, Qingdao University of Science and Technology, Qingdao 266042, P. R. China
b College of Environment and Chemical Engineering, Dalian University, Dalian 116622, P. R. China
c College of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China
*Email: zhouxin@dlu.edu.cn, luhuajiang@qust.edu.cn, qiujs@mail.buct.edu.cn
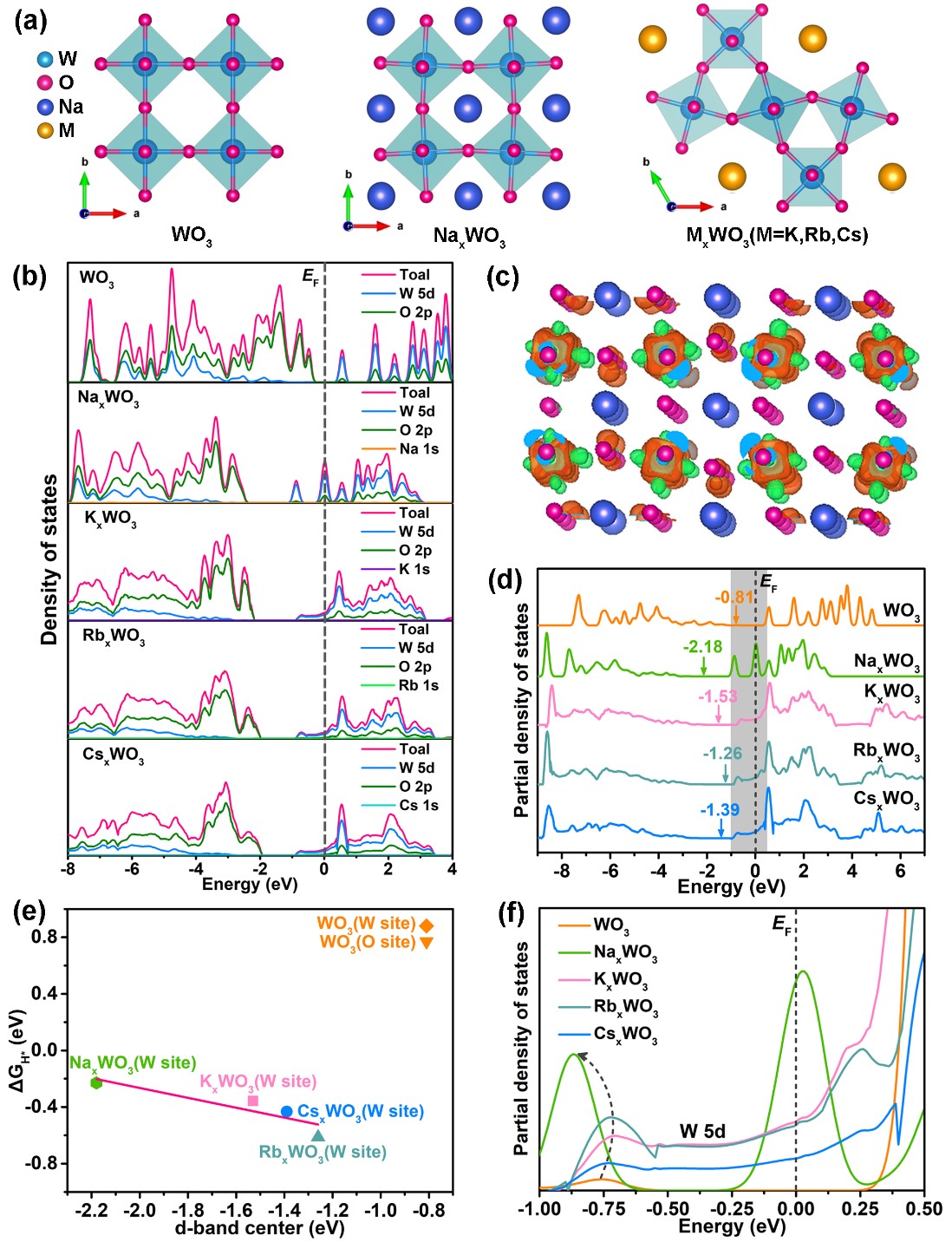
Abstract: Metal d-band configuration is decisive to the interaction of metals with reactants and intermediates, therefore often governs the catalytic activity. Herein, we for the first time demonstrate a family of alkali tungsten bronzes (MxWO3, M = Na, K, Rb, Cs and 0<x<1) for efficient hydrogen evolution reaction (HER) by modulating the W-d band configuration. Experimental and theoretical investigations on the newly-reported alkali tungsten bronzes reveal a linear relationship between the W-5d band center and the HER activity. Guided by this, the introduciton of sodium ions endows NaxWO3 with optimized electronic structure and W-d band configuration, leading to not only superior charge-transfer ability but also promoted H2O adsorption/dissociation kinetics and moderate hydrogen adsorption energy for wide-pH HER. As a result, NaxWO3 exhibits Pt-like activity in a wide-pH range, delivering an overpotential as low as 37, 48 and 226 mV at 10 mA cm-2 in acid, alkaline and neutral seawater, respectively. This work provides the first insight into the role of alkali metal ions in tungsten bronzes towasrds HER, which thus is expected to open up a new class of non-precious metal-based electrocatalysts for the HER and other energy storage and conversion reactions.
Keywords: alkali tungsten bronze, porous octahedron, d-electron, wide-pH, hydrogen evolution
https://doi.org/10.1016/j.nanoen.2024.109442