Lang Xiao, Wanqing Yu, Jing Liu *, Shankui Luan, Wenyu Pei, Xuejing Cui, Luhua Jiang*
College of Materials Science and Engineering, Qingdao University of Science and Technology,
Qingdao, Shandong 266042, China.
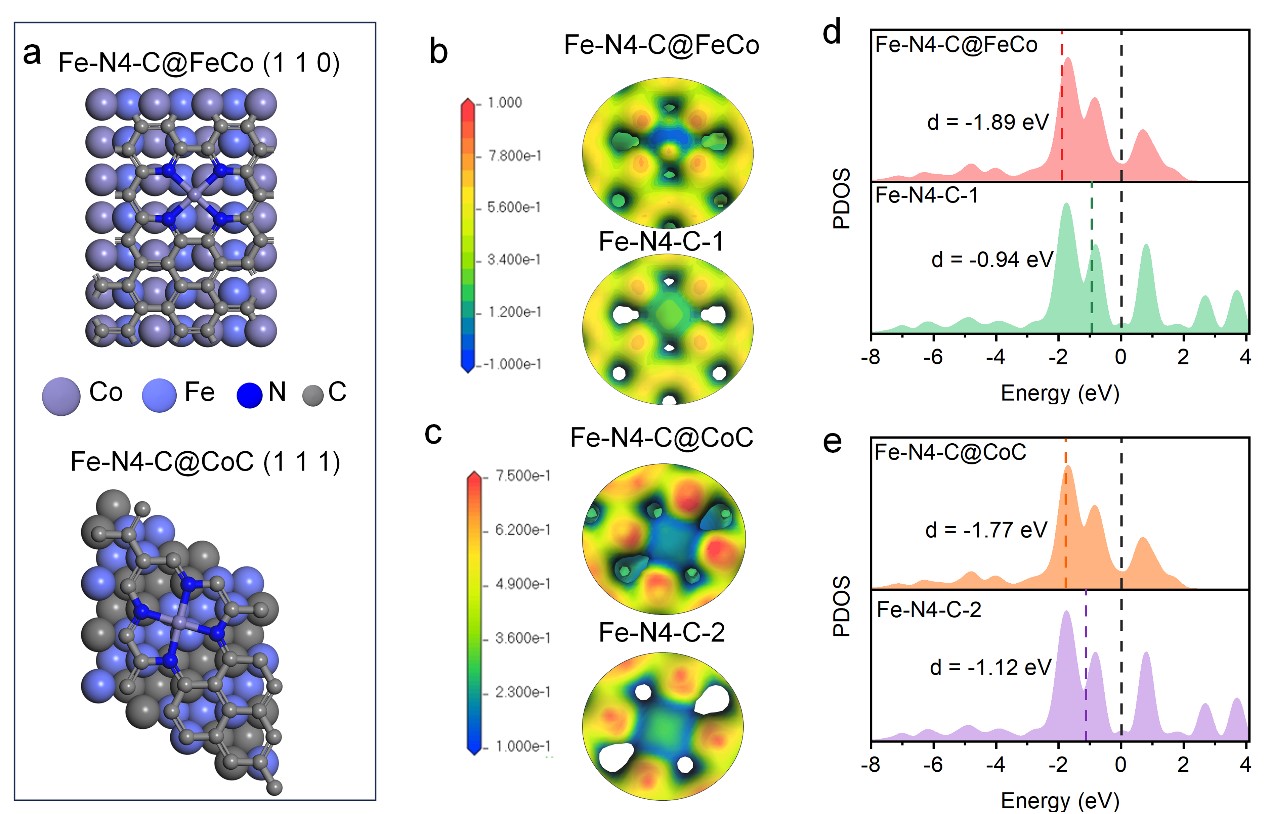
Abstract: Developing an efficient and stable non-precious metal catalyst (NPMC) for oxygen reduction reaction (ORR) is crucial for Zn-air batteries. Herein, we report a super-molecule self-scarifying template and confinement pyrolysis strategy to obtain an efficient ORR catalyst of well-dispersed Co3Fe7/CoCx heterostructure nanoparticles encapsulated by nitrogen-doped carbon nanotubes (Co3Fe7/CoCx@N-CNT). The as-synthesized Co3Fe7/CoCx@N-CNT catalyst exhibits outstanding ORR activity, with a half-wave potential of 0.88 V versus a reversible hydrogen electrode (vs. RHE) and good stability. The Zn-air battery based on the Co3Fe7/CoCx@N-CNT cathode achieves a peak power density of 265 mW cm-2 and extra-long durability over 200 hours, which is superior to most of the reported NPMCs and even the Pt/C counterpart. The physical characterization and electrochemical poisoning experiments discover that Co3Fe7/CoCx nanoparticles in the core along with pyridine N and Fe-Nx hosted in the carbon nanotube all act as active sites for the ORR. Further theoretical calculation demonstrates charge redistribution between the Co3Fe7/CoCx nanoparticles and the Fe-Nx carbon overlayers downshift d-band center of Fe and optimize the adsorption, which boosts the ORR kinetics. This work provides an effective strategy to synthesize non-precious metal ORR catalysts with multiple active sites and highlights the synergistic role of encapsulated nanoparticles and carbon support.
Keywords: Heterostructure; Oxygen reduction reaction; Confined pyrolysis; Zn-air battery; Synergistic catalysis
*Corresponding authors: liuj955@qust.edu.cn; luhuajiang@qust.edu.cn (L. Jiang)
https://doi.org/10.1016/j.jcis.2023.11.034