Wanqing Yu, Jing Liu*, Hao Hu, Zeyu He, Xuejing Cui, Luhua Jiang*
College of Materials Science & Engineering, Qingdao University of Science & Technology, Qingdao, 266042, P.R. China
E-mail: liuj955@qust.edu.cn; luhuajiang@qust.edu.cn
Abstract:
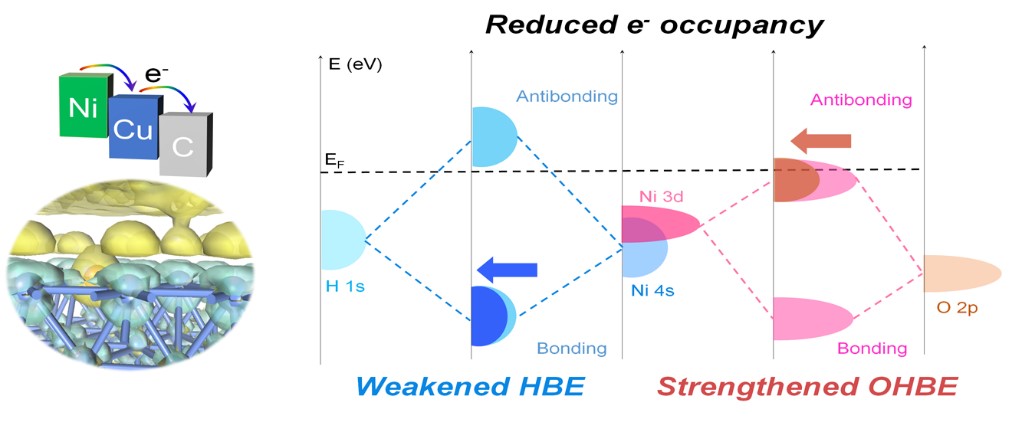
Nickel-based catalysts are recognized as a promising alternative to platinum-based catalysts for the alkaline hydrogen oxidation reaction (HOR), yet suffer from poor stability and relatively low activity. Herein, we report a nitrogen ligand-assisted approach to encapsulate nickel-copper nanoparticles within few carbon layers, and by modulating the core and shell components/structure, the charge distribution in the nanostructures could be finely regulated. The optimized Ni93Cu7@NC catalyst exhibits outstanding HOR activity with an intrinsic activity of 61.0 μA cm-2 and excellent stability, which is among the most advanced Ni-based HOR catalysts. Notably, an alkaline exchange membrane fuel cell utilizing this catalyst achieves a peak power density of 381 mW cm–2 and maintains stable at 100 mA cm-2 for over 24 hours. Experimental and theoretical investigations unveil that the electron re-distribution at the interface of NiCu core and nitrogen-doped carbon reduces the electron occupancy in Ni 4s-H 1s bonding orbitals and Ni 3dz2/yz-O 2p antibonding orbitals, leading to a weakened hydrogen binding energy and enhanced hydroxide binding energy. Consequently, the limiting energy for the HOR is reduced following a bifunctional mechanism on the Ni93Cu7@NC. This work provides a core-shell co-modulation strategy to accurately regulate the electronic structure of transition metals to design robust catalysts.
DOI: 10.1002/adfm.202315062