Lang Xiaoƚ, Wanqing Yuƚ, Jing Liu*, Shankui Luan, Shengchang Li, Xuejing Cui, Luhua Jiang*
College of Materials Science and Engineering, Qingdao University of Science and Technology, Qingdao, Shandong 266042, China.
Abstract:
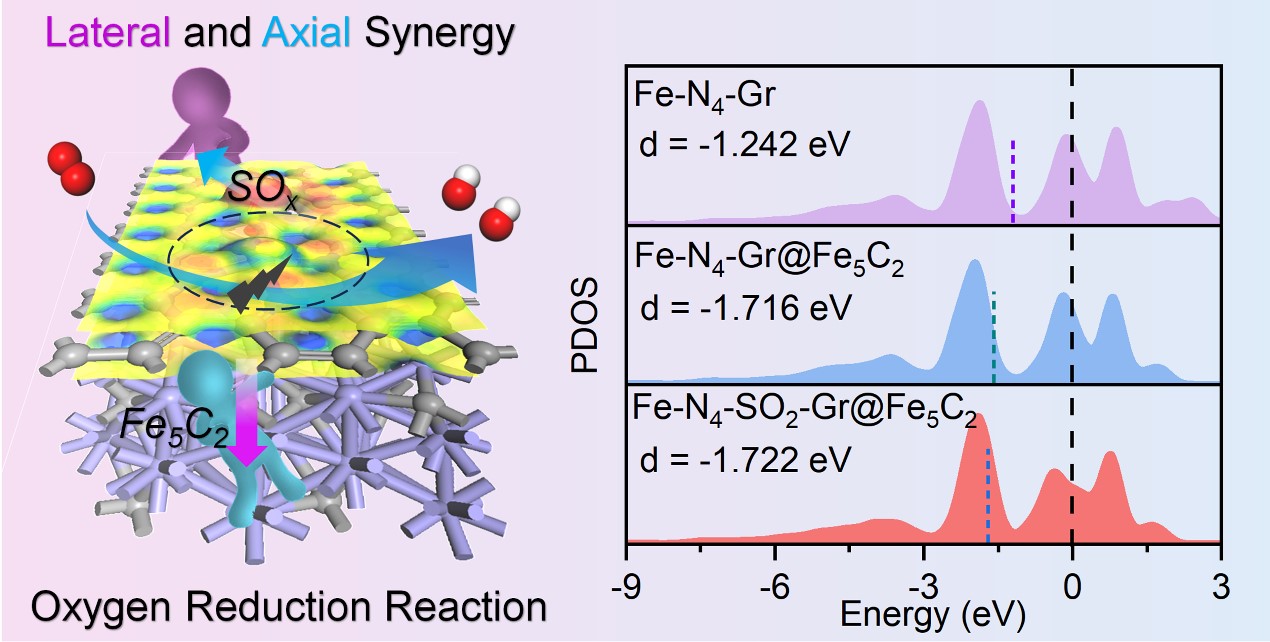
A novel ORR electrocatalyst, consisting of an N, S co-doped carbon shell encapsulating Fe5C2 nanoparticles, is developed through self-assembled supramolecular structures and controlled pyrolysis. The resulting Fe5C2@SNC catalyst exhibits exceptional electrocatalytic performance, with a high half-wave potential (E1/2) of 0.86 V, comparable to commercial Pt/C. The distinctive core-shell structure contributes to excellent stability, demonstrating a 92.2% current maintenance after 20 hours of continuous chronoamperometry testing. In Zn-air battery applications, the catalyst achieved a peak power density of 233 mW cm-2, surpassing its Pt/C counterpart. Combining the experiments and density functional theory (DFT) calculations, synergistic effects of axial Fe5C2 nanoparticles and laterally SOx-functionalized Fe-Nx carbon planes within Fe5C2@SNC have been comprehensively unveiled. The electron-withdrawing nature of sulfur leads to charge redistribution, particularly on N sites proximal to the SOx group. Additionally, the axial Fe5C2 nanoparticles have precisely modulated the d-band center of the Fe5C2@SNC catalyst, optimizing oxygen intermediate adsorption and enhancing ORR activity. This work highlights the understanding and harnessing of synergistic catalysis via a controllable core-shell structure, providing an effective way for developing highly efficient and stable electrocatalysts for energy conversion and storage applications.
Keywords: Oxygen reduction reaction; sulfur doping; synergy; Zn-air battery; non-noble catalyst
ƚ These authors have contributed equally to this work.
* Corresponding authors: liuj955@qust.edu.cn; luhuajiang@qust.edu.cn (L. Jiang)
https://doi.org/10.1021/acssuschemeng.4c00286