Jie Gao, Wanqing Yu, Jing Liu *, Lishuai Qin, Haodong Cheng, Xuejing Cui, Luhua Jiang *
Electrocatalysis & Nanomaterial Laboratory, College of Materials Science & Engineering, Qingdao University of Science & Technology, Qingdao, 266042, China.
ABSTRACT:
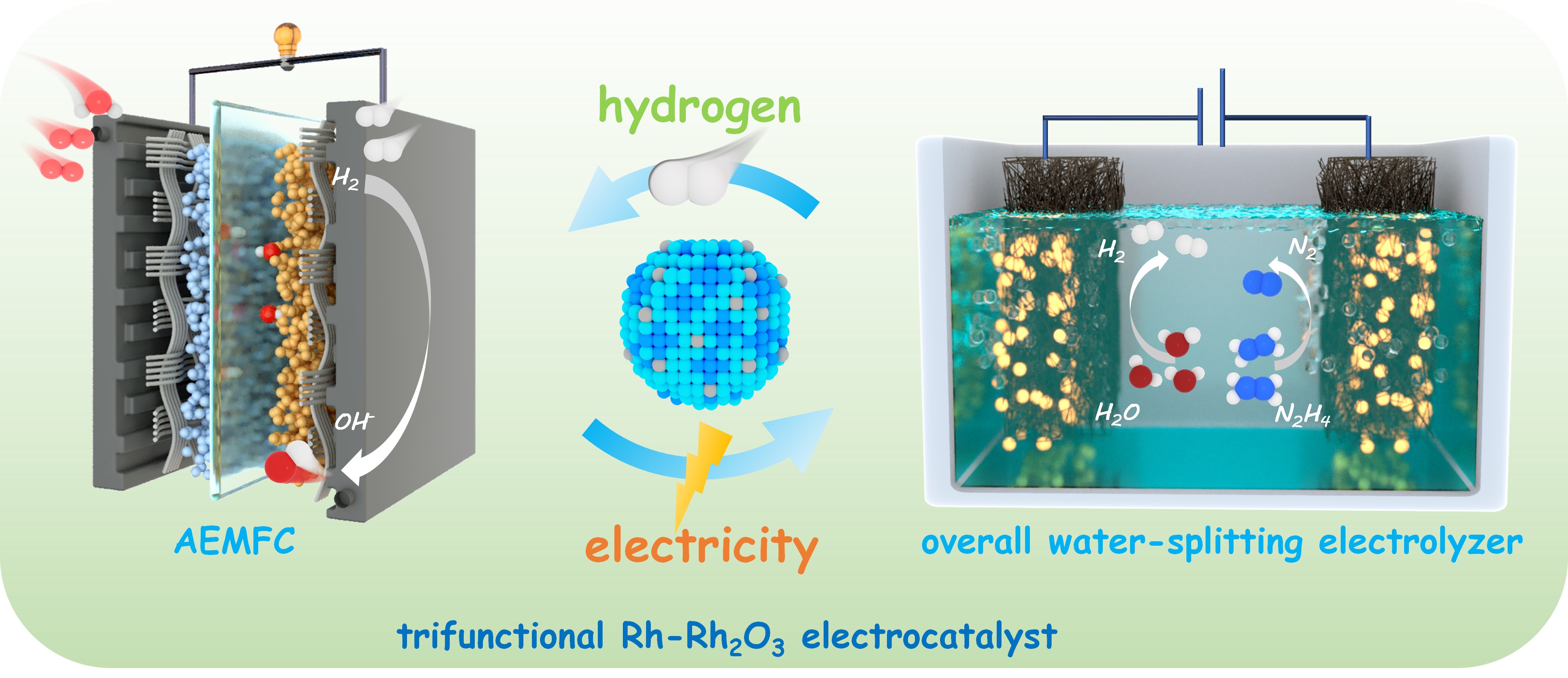
Hydrogen production and utilization efficiency relies heavily on electrocatalysts. In this work, a Rh-Rh2O3 cluster with oxygen vacancies (Rh-OV-Rh2O3) is prepared as a tri-functional electrocatalyst for hydrogen oxidation reaction (HOR), hydrogen evolution reaction (HER), and hydrazine oxidation reaction (HzOR), involved in hydrazine-assisted water electrolysis for hydrogen production and a hydrogen fuel cell. The optimized Rh-Rh2O3/C-400 catalyst exhibits a mass activity of 2.29 mA μgRh-1 for HOR, with low potentials of 12 mVRHE for HER and 31 mVRHE for HzOR at 10 mA cm-2 in alkaline media, superior to the commercial Pt/C. It also displays promising performance in practical devices, i.e., the H2-O2 anion-exchange-membrane fuel cell delivers a high peak power density of 0.66 W cm-2, and the hydrazine-assisted water splitting electrolyzer based on Rh-Rh2O3/C-400 symmetric electrodes exhibits a low electrolysis voltage of 0.161 V at 0.1 A cm-2. Mechanistic studies reveal that the optimal hydrogen binding energy on Rh-OV-Rh2O3 contributes to the excellent catalytic activities in HOR, HER, and HzOR. This work not only develops a superior tri-functional Rh-based catalyst, but also highlights the importance of local chemical environments in surface, which especially for those reaction involving multi-intermediates governs the catalytic performance by manipulating the adsorption states/strength of intermediates/reactants.
Keywords: hydrogen electrode reaction; hydrazine oxidation reaction; tri-functional electrocatalyst; Rh-Rh2O3 cluster; oxygen vacancy
*Corresponding authors: liuj955@qust.edu.cn; luhuajiang@qust.edu.cn (L. Jiang)
https://doi.org/10.1016/j.jcis.2024.03.095