Xiaolei Lia, Chen Songa, Shuyuan Yangc, Qisen Jiaa, Xuejing Cuia, Guangbo Liua,*, Xin Zhoub,c,*, and Luhua Jianga,*
a College of Materials Science and Engineering, Qingdao University of Science and Technology, Qingdao 266042, P. R. China
b Interdisciplinary Research Center for Biology and Chemistry, Liaoning Normal University, Dalian 116029, P. R. China
c College of Environment and Chemical Engineering, Dalian University, Dalian 116622, P. R. China
* Corresponding authors: liugb@qust.edu.cn; zhouxin@dlu.edu.cn; luhuajiang@qust.edu.cn
Abstract:
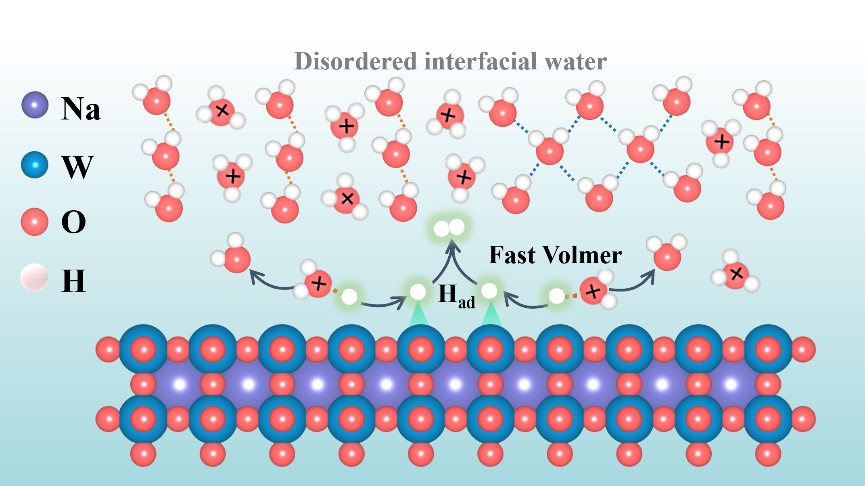
Understanding the role of cations within the catalysts in the interfacial water behavior at the electrolyte/catalyst interface is pivotal importance for designing advanced catalysts toward hydrogen evolution reaction (HER), which remains obscure and requires deep probing. Herein, we demonstrate the first investigation of interfacial water behavior on surface of series ofsodium tungsten bronzes (NaxWO3, 0 < x < 1) by using electrochemical, operando spectroscopic and theoretical characterizations, aiming to gain the fundamental insight into the role of sodium ion in the interfacial water structure and subsequent HER at the NaxWO3/electrolyte interface. Our integrated studies indicate that the Na ions significantly enrich the electronic state of WO6 octahedrons in NaxWO3, which leads to the regulated electronic and atomic structures, endowing NaxWO3 with disordered interfacial water network containing more isolated H3O+ and subsequently moderate H* adsorption to speed the Volmer step at the NaxWO3 surface, thus boosting the HER. Consequently, the intrinsic HER activities achieved on those NaxWO3 are tens of times higher than that on WO3. Particularly, it is found that Na concentration x = 0.69 endows NaxWO3 with the highest intrinsic HER activity, and the resultant Na0.69WO3 with unique porous octahedral structure exhibits a low overpotential of only 64 mV at current density of 10 mA cm-2 in acidic electrolyte. This study provides the first insight into the cation-dependent interfacial water behavior induced by the cations within catalyst and establishes the interfacial water-activity relationship of HER, thus allowing for the design of more advanced catalyst with efficient interfacial structures towards HER.
Keywords:Sodium tungsten bronze; sodium-ion; hydrogen evolution; interfacial water; structure-activity relationship