Jing Liu, Wanqing Yu, Mengdi Wang, Jie Gao, Xuejing Cui, Luhua Jiang*
College of Materials Science & Engineering, Qingdao University of Science & Technology, Qingdao, 266042, P.R. China
Abstract:
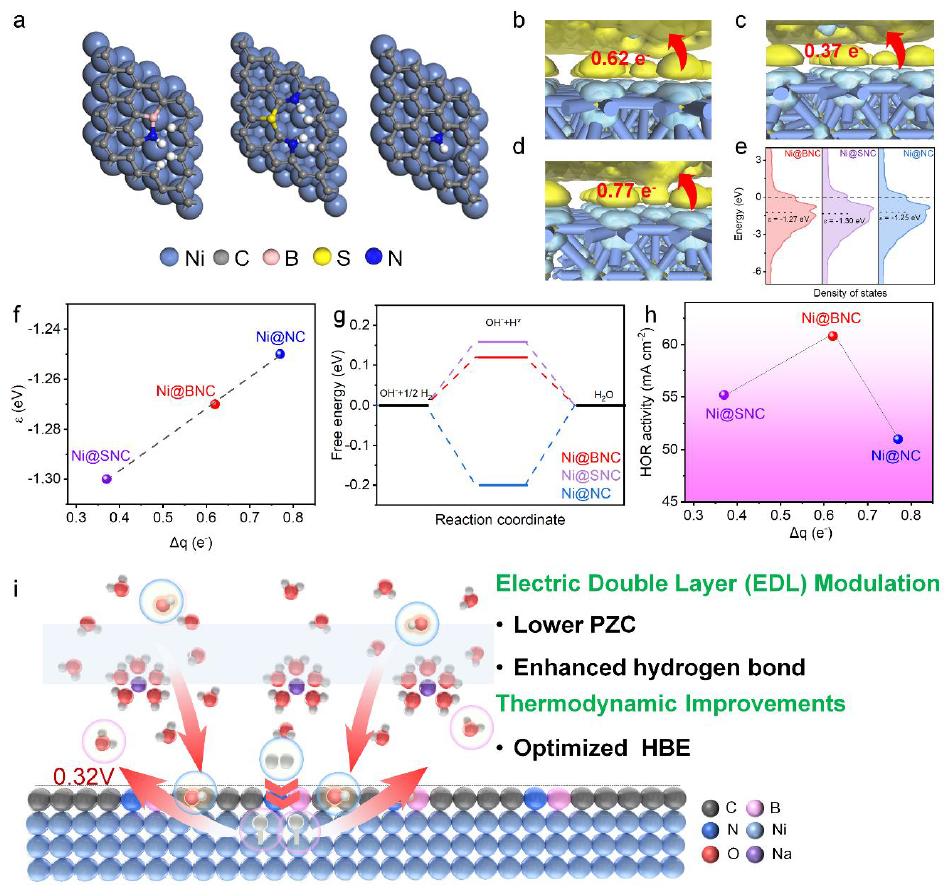
Tuning metal - support interaction by encapsulating metal nanoparticles within carbon shells has emerged as a strategy to enhance alkaline hydrogen oxidation reaction (HOR) catalysts. However, the precise impact of the carbon shell on catalytic activity remains to be fully understood, yet is crucial to guide the rational design of core - shell structured catalysts. In this study, with a sequence of well - designed Ni catalysts coated by heteroatom - doped carbon layers (including Ni@BNC, Ni@SNC and Ni@NC), we discover a volcano - type relationship between the electron polarization degree and the HOR activity. Experimental and theoretical analyses show heteroatom doping adjusts the carbon layer’s electron - withdrawing ability, modulating the Ni core’s d - band center and hydrogen binding energy (HBE). Additionally, heteroatom doping shifts the potential of zero charge (PZC) negatively and enhances hydrogen bond connectivity, facilitating hydroxyl ion transfer. As a result, Ni@BNC achieves optimal HBE and enhanced water adsorption, placing it at the volcano summit for HOR activity. This study establishes a delicate volcano - relationship between the electron polarization degree and the HOR activity of core - shelled catalysts, by shedding lights on the underlying determining factors, both the d - band center of metals and the surface PZC, related tightly with the HBE and hydrogen bond connectivity/water adsorption.
https://doi.org/10.1021/acscatal.5c00053