Guangbo Liua, Chen Songa, Xiaolei Lia, Qisen Jiaa, Pengfei Wua, Zhihao Loua, Yuanshuo Maa, Xuejing Cuia, Xin Zhoub,c,*, and Luhua Jianga,*
a College of Materials Science and Engineering, Qingdao University of Science and Technology, Qingdao 266042, P. R. China
b Interdisciplinary Research Center for Biology and Chemistry, Liaoning Normal University, Dalian 116029, P. R. China
c College of Environment and Chemical Engineering, Dalian University, Dalian 116622, P. R. China
*Email: zhouxin@dlu.edu.cn; luhuajiang@qust.edu.cn
Keywords: Defect-rich; high-entropy alloy; hydrogen evolution reaction; interfacial water; in-situ FTIR
Abstract:
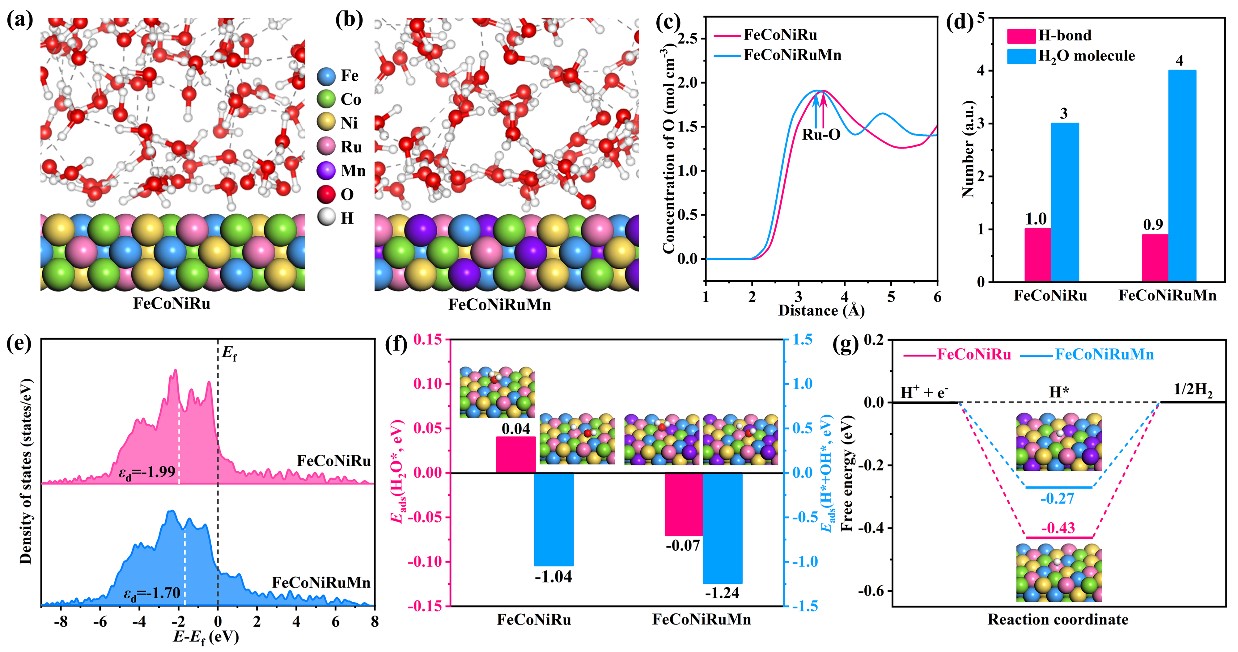
Water molecules are the most important participant in hydrogen evolution reaction (HER) under alkaline water/seawater conditions, while effectively activating the rigid interfacial water and promoting the HER kinetics remain a great challenge. Herein, we construct a defect-rich FeCoNiMnRu high-entropy alloy (HEA) by rapid Joule-heating approach, which could significantly reform the structure and dynamics of interfacial water and consequently boost the HER activity. In-situ Fourier transform infrared (FTIR) spectra and ab initio molecular dynamics (AIMD) calculations confirm that the constructed FeCoNiMnRu HEA endows a fast conversion of interfacial water from strong H-bonded water to free water, which increases the availability and activity of H2O* at the active sites, and thereby facilitating the HER process. Consequently, the developed defect-rich FeCoNiMnRu HEA exhibits excellent activity with overpotentials of only 37 and 35 mV at 10 mA cm-2 in alkaline water and seawater electrolytes, respectively, which are surpassing that of commercial Pt/C. This work discloses the behavior and structure of interfacial water on HEA and provides new insights into the design of advanced HEA-based catalysts towards HER.