Jinxiu Wang a, Zhen Liu a, Wen Jin a, Yongxiao Tuo b*, Yan Zhou c, Shanshan Zhou a, Tingting Gong a, Jiamei Li a, Yuting Ni a, Min Wang a*, Luhua Jiang a*
a College of Materials Science & Engineering, Qingdao University of Science & Technology, Qingdao, 266042, P.R. China.
b State Key Laboratory of Heavy Oil Processing, College of New Energy, China University of Petroleum (East China) Qingdao, Shandong 266580, P. R. China.
c Phonon Engineering Research Center of Jiangsu Province, Center for Quantum Transport and Thermal Energy Science, Institute of Physics Frontiers and Interdisciplinary Sciences, School of Physics and Technology, Nanjing Normal University, Nanjing 210023, China.
*Corresponding authors: E-mail: yxtuo@upc.edu.cn; wmin@qust.edu.cn;luhuajiang@qust.edu.cn
Keywords: CO2 electrochemical reduction, In-situ characterization,Cu2OSO4@CuO catalyst,In-situ Evolution,DFT calculations
Abstract
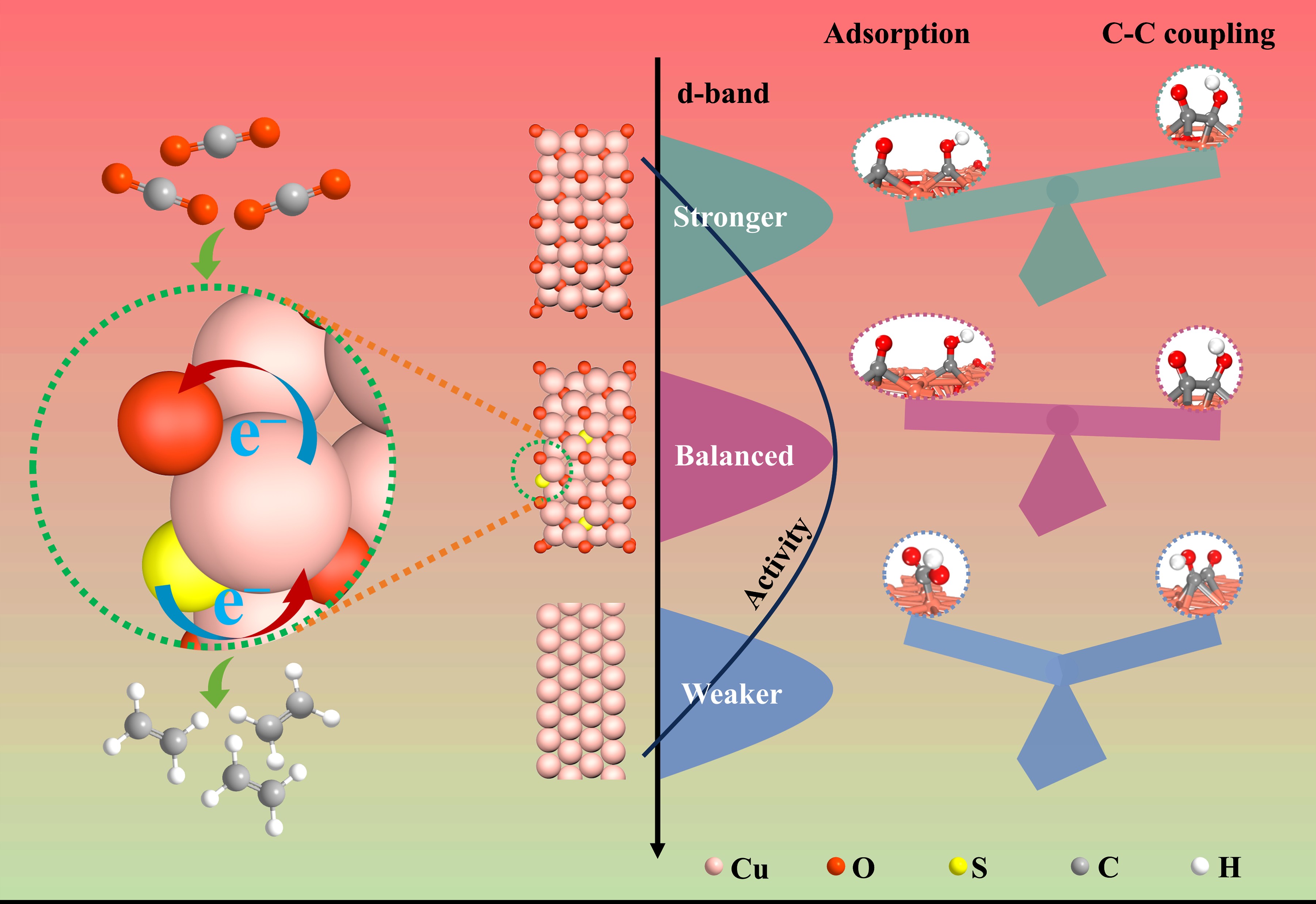
Real-time tracking the dynamic structure reconstruction of Cu-based catalysts during CO2 reduction reaction (CO2RR) and identifying the active sites of catalysts are essential for fundamental understanding on electrocatalysis and thereby rational design of catalysts. However, the exact relationship between structure reconstruction and CO2RR performance remains poorly understood, thus bringing great challenges to rationally designing catalysts and understanding the reaction mechanism. Herein, by virtue of comprehensive in-situ and ex-situ studies, the dynamic structure reconstruction of Cu2OSO4@CuO is elucidated, and it is demonstrated that Cu2OSO4@CuO evolves to S-incorporated Cu2O@Cu (S-Cu2O@Cu). In-situ surface-enhanced infrared absorption spectroscopy (SEIRAS) and density functional theory (DFT) calculations reveal that Cu2O is conducive to the generation of *CO, while the incorporation of S downshifts the d-band center of Cu in Cu2O, facilitating desorption and sequent migration of *CO and *COH to undergo C-C coupling. Consequently, a maximal FE(C2+) of as high as 88% with a partial current density of –609 mA cm–2 is achieved for the reconstructed Cu2OSO4@CuO, which outperforms the state-of-the-art Cu-based catalyst. This work not only highlights the significant role of the incorporation of sulfur in enhancing the CO2RR activity over Cu2O, but also provides a feasible strategy to obtain stabilized Cu2O electrocatalyst for CO2RR.