Shengchang Li, Jing Liu*, Shuo Li, Jiansheng Song, Xuejing Cui, Luhua Jiang*
College of Materials Science and Engineering, Qingdao University of Science and Technology, Qingdao, Shandong 266042, China.
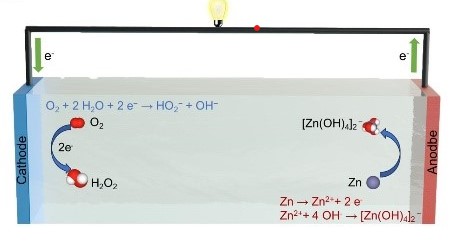
Abstract:The synthesis of hydrogen peroxide (H2O2) via the two-electron oxygen reduction reaction (2e- ORR) is a promising alternative to the conventional anthraquinone process. Herein, we report an integrated system for catalyzing the oxygen reduction reaction (ORR) in zinc-air batteries (ZABs) using a Co/N co-doped carbon nanofiber electrocatalyst (CoNC@CNF-900/OPT) fabricated via electrospinning. The optimized CoNC@CNF-900/OPT achieved a half-wave potential (E1/2) of 0.786 V and an H2O2 selectivity of 62.5% at 0.3 V vs. RHE. In an H-type electrolytic cell, the catalyst exhibited an H2O2 production rate of 3605 mmol gcat-1 h-1. When employed as the cathode in ZABs, the system delivered a peak power density of 179 mW cm-2 and maintained 93.5% initial activity after 24 h of operation at 20 mA cm-2. Furthermore, in a flow-state ZAB configuration, the catalyst delivered an integrated performance with a peak power density of 61.97 mW cm-2 and an H2O2 yield of 73.79 mmol g-1 h-1. This work highlights the potential of ZABs for in situ H2O2 production, thereby providing a technological foundation for developing multifunctional devices that integrate energy supply and chemical synthesis.
Keywords: oxygen reduction reaction; Zn-air batteries; hydrogen peroxide; electrocatalysis; non-precious electrocatalyst
*Corresponding authors: liuj955@qust.edu.cn (J. Liu); luhuajiang@qust.edu.cn (L. Jiang).