Mengdi Wang1, Jing Liu1*, Nuo Sun1, Li Wang1, Zhangrong Lou2, Xuejing Cui1, Luhua Jiang1*
1College of Materials Science & Engineering, Qingdao University of Science & Technology, Qingdao, 266042, P.R. China
2Dalian University of Technology, Dalian, 116024, P.R. China
Abstract:
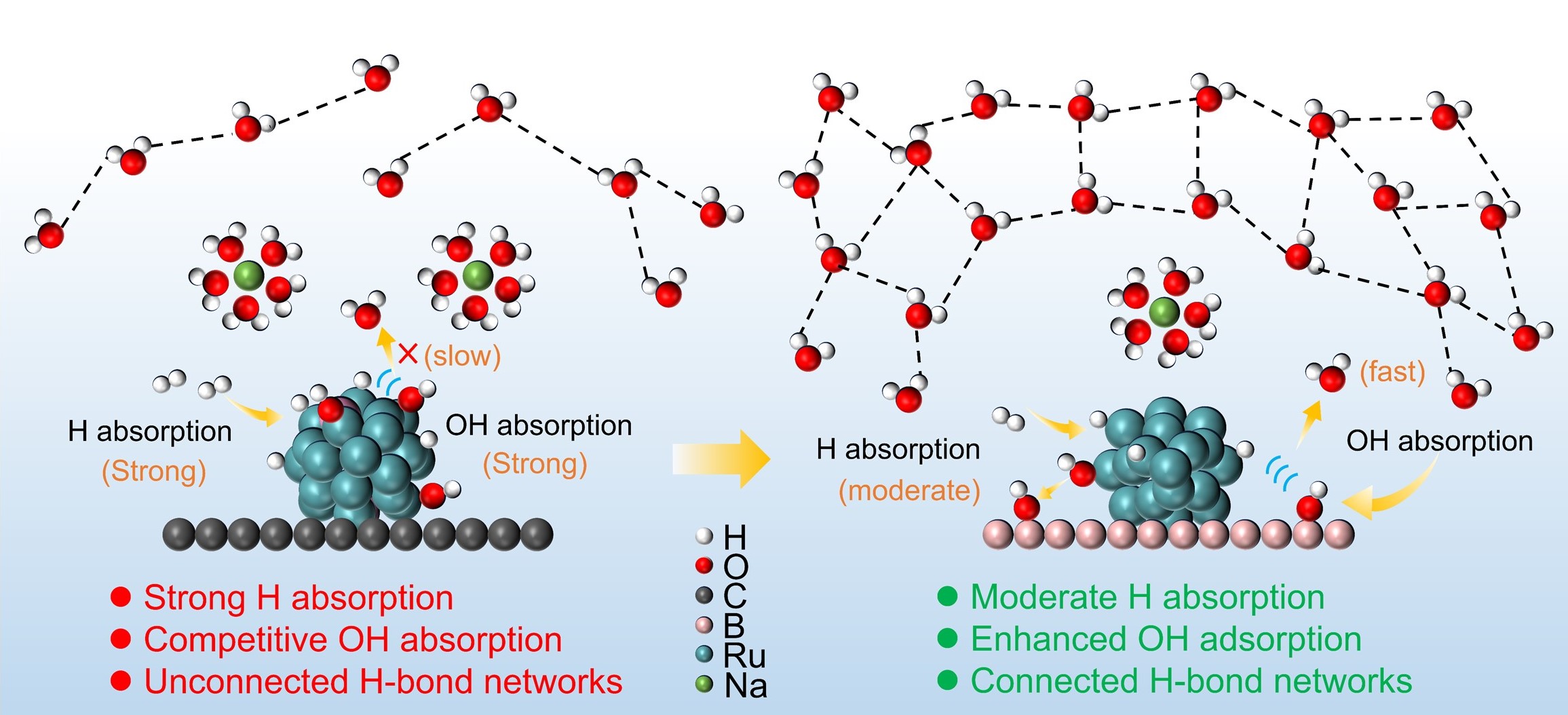
Ruthenium (Ru) has long been handicapped by its poor activity and stability for the alkaline hydrogen oxidation reaction (HOR). Herein, we confine Ru clusters with two-dimensional (2D) borophene (Ru-B-C-350) to activate strong metal-support interaction via d-p orbital hybridization. Ru-B-C-350 exhibits superior alkaline HOR performance with mass activity and specific activity of 2.16 mA/μgRu and 0.56 mA/cm2, respectively, along with remarkable stability of only a 3% decrease in activity after 10,000 cycles. The anion exchange membrane fuel cell (AEMFC) with the Ru-B-C-350 anode delivers a peak power density of up to 792 mW/cm2, outperforming the Pt/C counterpart. Theoretical calculations suggest that the enhanced coupling between the p orbitals of the 2D borophene and the d orbitals of Ru optimizes hydrogen adsorption on the Ru sites and OH adsorption on the boron sites. Moreover, in-situ surface-enhanced infrared absorption spectroscopy (SEIRAS) demonstrates that the 2D borophene significantly improves the connectivity of the hydrogen bonding network in the electric double layer, accelerating the HOR kinetics. This work offers insights into enhancing HOR efficiency through the confinement effects of metal-2D borophene, which leverages d-p orbital coupling and interfacial microenvironment, providing a promising strategy for designing high-performance HOR catalysts.
Keywords: hydrogen oxidation reaction, ruthenium, two-dimensional (2D) borophene, d-p orbital coupling, interfacial water
*Corresponding authors: E-mail: liuj955@qust.edu.cn; luhuajiang@qust.edu.cn