Hao Hu1, Nuo Sun1, Jie Gao1, Li Wang1, Zhangrong Lou2, Xuejing Cui1, Jing Liu1*, Luhua Jiang1*
1 College of Materials Science & Engineering, Qingdao University of Science & Technology, Qingdao, 266042, P.R. China
2 Dalian University of Technology, Dalian, 116024, P.R. China
Abstract:
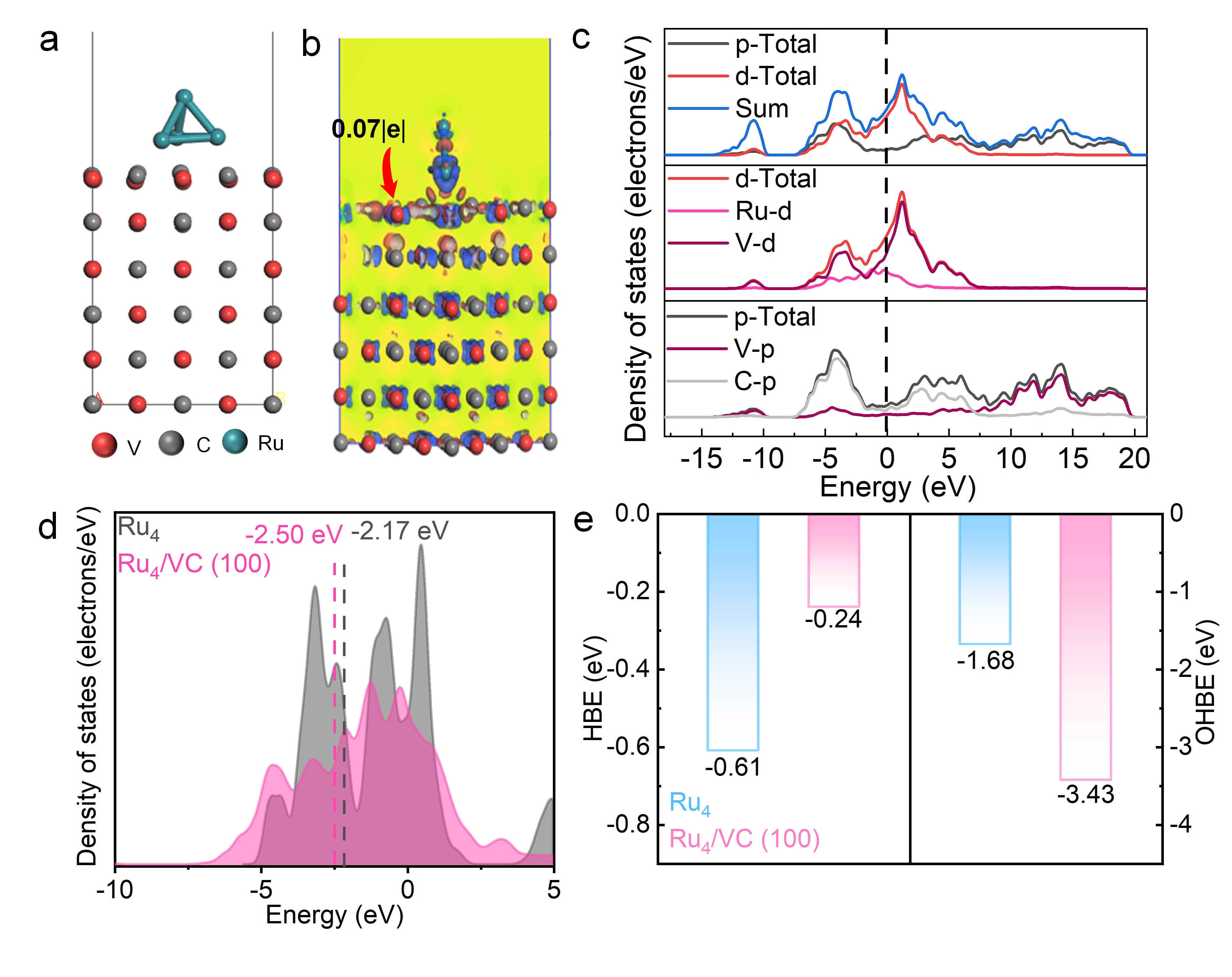
Ru as a promising candidate for alkaline hydrogen oxidation (HOR) and hydrogen evolution reactions (HER), is limited by its intrinsic catalytic activity due to the mismatched adsorption for intermediates as well as the insufficient optimization of the interfacial water structure in the electric double layers. Herein, we report a Ru-vanadium carbide (VC) heterostructure catalyst supported on nitrogen-doped carbon, namely Ru/VC@NC, which demonstrates remarkable hydrogen electrocatalysis performance, with a mass activity of 0.87 mA μgRu⁻¹ and a specific activity of 0.63 mA cm⁻² for HOR, along with an impressively low overpotential of just 24 mV at 10 mA cm⁻² for HER, substantially outperforming Pt-C (29 mV) and Ru-XC (42 mV). Comprehensive experimental and theoretical investigations reveal that the electronic structure of Ru/VC is effectively modulated, due to the orbital coupling of Ru d-orbital and VC d/p orbitals, optimizing the adsorption behavior of reaction intermediates. Specifically, the downshifted d-band center of Ru suppresses excessive hydrogen binding energy (HBE), while the V sites in VC enhance hydroxide binding energy (OHBE). Combined with the more interconnected H-bonding network at the electrical double layer (EDL) that facilitates intermediate transport revealed by in-situ surface-enhanced infrared absorption spectroscopy (SEIRAS) these features collectively contribute to the improved hydrogen electrocatalysis. This work highlights the advantage of interfacial engineering in strengthening orbital coupling and synergistically tuning both intermediate adsorption and the reaction microenvironment, to enhance the catalytic activity.
https://doi.org/10.1016/j.cej.2025.167317